The 340B program plays a crucial role in enabling healthcare providers to deliver affordable medications to underserved communities. However, its rapid expansion has brought complex operational and compliance challenges, which can result in severe financial penalties, reputational damage, and even loss of program eligibility.
To avoid non-compliance or potential implementation challenges, a thorough understanding of the program is essential. Therefore, in this blog, we will discuss the 340B program in detail, including how it works, who qualifies for it, and the major stakeholders involved.
Furthermore, towards the end, we have mentioned recommendations that can help you strengthen your 340B compliance. First, let’s start with a brief explanation of the 340B program.
Understanding the 340B Program
The 340B Drug Pricing Program aims to reduce the cost of prescription drugs for eligible healthcare providers and their patients. Additionally, it requires drug manufacturers to sell outpatient drugs at reduced prices to covered entities (we will discuss covered entities later in this blog). Currently, thousands of covered entities are participating in this program. As a report, released earlier this year by the chairman of the US Senate Health, Education, Labor, and Pensions Committee, noted that over 60,000 covered entities were participating in the program (as of February 2025). However, the HRSA FY 2023 audit results (finalized in January 2025) showed 63% of audited covered entities had at least one compliance finding, meaning they failed to meet at least one program requirement. Let us tell you how this program works to help you gain clarity on its structure, eligibility criteria, and operational requirements.
How does the 340B Program Work? Comprehending Who Qualifies, Stakeholders & Impact
340B mandates the drug manufacturers to sell discounted drugs to federally qualified health centers and disproportionate share hospitals. In simple terms, this program offers discounted prescription drugs to eligible healthcare providers, known as covered entities, to assist them in serving low-income and uninsured patients.
Furthermore, this pricing model enables healthcare facilities, hospitals, and clinics to generate savings that can be reinvested in patient care programs, thereby expanding access to care for underserved communities. Additionally, the infographic below will help you better understand how this program works.
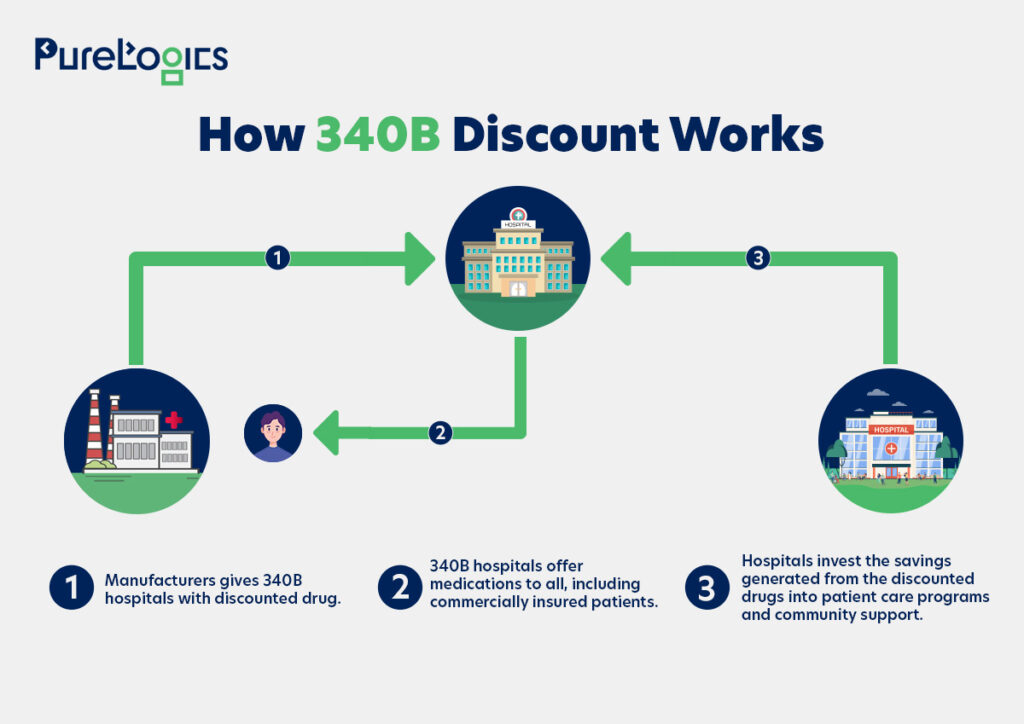
Who Qualifies for the 340B Program?
The entities eligible for the 340B program participation are:
- Children’s hospitals
- Rural referral centers
- Critical access hospitals
- Sole community hospitals
- Free-standing cancer hospitals
- Some community health centers
- Disproportionate share hospitals (DSH)
- Federally Qualified Health Centers (FQHCs)
Additionally, to participate, eligible facilities must demonstrate that they serve a high proportion of low-income or uninsured patients.
Stakeholders of the 340B Program
The U.S. Department of Health and Human Services currently manages the 340B program. But under the proposed 2026 budget, oversight would be transferred to the Centers for Medicare & Medicaid Services. Other stakeholders include:
- 340B covered entities: Healthcare organizations eligible to purchase discounted 340B medications. They use the savings to expand services and better serve patients.
- Pharmacy Benefit Managers (PBMs): Third-party companies that manage prescription drug benefits for health plans. In the 340B program, they help process and reimburse prescriptions.
- Third-Party administrators (TPAs): Vendors or organizations that manage 340B program tasks for covered entities, including enrollment, prescription tracking, compliance, and reporting.
- 340B drug manufacturers: Pharmaceutical companies that participate in the 340B program by offering discounted medications to eligible healthcare organizations. To include their drugs under Medicaid, they must join the Medicaid Drug Rebate Program and agree to the program’s pricing requirements.
- 340B contract pharmacies: External pharmacies, like CVS or Walgreens, that dispense 340B drugs to eligible patients on behalf of covered entities.
In addition to helping covered entities offer discounted medications, it also enables hospitals and clinics to reinvest their savings in patient services, programs, and infrastructure. Although the correct implementation of this program is necessary to leverage its full advantages, an expert healthcare software development company like PureLogics can help you with that.
Moreover, failure to follow 340B rules can have serious repercussions for covered entities and manufacturers as non-compliance may result in financial penalties, including repayments for overcharges or improperly claimed discounts, as well as duplicate discounts (when a drug is discounted under both the 340B program and the Medicaid Drug Rebate Program), which can lead to termination of program eligibility.
Partner with Experts in 340B Program Management
Leverage PureLogics’ decades of experience to ensure compliance, streamline operations, and maximize program benefits.
Recommendations to Strengthen 340B Compliance
To address the increasing complexity of the 340B program, healthcare organizations must rethink how technology and data interact. Whether it is a pharmacy, PBM, or third-party administrator, enhancing digital infrastructure is key to ensuring compliance and accuracy.
Build an Integrated 340B Ecosystem For All Stakeholders
Disparate systems are a significant source of errors and audit failures, as covered entities, TPAs, and PBMs should adopt an integrated data environment that connects pharmacy claims and EHR systems. The unified view allows accurate eligibility validation and transaction transparency across all 340B partners.
Use AI-Powered Auditing to Detect Compliance Risks
AI and machine learning can help automate ongoing compliance reviews, identifying duplicate discounts, diversion risks, and pricing anomalies before they become HRSA findings. PBMs and TPAs can especially benefit from predictive analytics that flag suspicious rebate or chargeback activity in near real-time.
Automate Reporting for Audit Readiness
Hospitals should move away from manual audit preparation, as automated workflows and real-time dashboards can simplify responses to HRSA and manufacturer audits. Whilst the PBMs and contract pharmacies can use API-based integrations to push verified data directly into audit repositories.
Streamline Your 340B Solutions with AI
Our AI engineers design modern healthcare software to simplify 340B compliance, administration, and financial management.
Strengthen Collaboration Via Shared Portals
Data mismatches and disputes between PBMs, TPAs, and covered entities remain a significant compliance risk. However, shared portals with role-based access, audit trails, and data reconciliation tools can reduce disputes and align records. In addition, to correct reimbursement across all parties.
Continuously Update Systems to Stay Compliant
The 340B participants must ensure that their systems are regularly updated with HRSA rules, manufacturers’ restrictions, and state PBM laws (that are continuously evolving).
Automation should extend to compliance monitoring so that any change in regulation or pricing logic immediately triggers workflow and policy updates.
Full-time Expert 340B Program Management
Healthcare systems often attempt to manage 340B compliance independently or manually, which can lead to loopholes that result in HRSA audit findings, duplicate discount errors, diversion issues, and potential financial penalties. This jeopardizes the program’s eligibility and the sustainability of savings. Therefore, going for 340B software development can ensure accurate regulatory compliance reporting.
340B Program: Recent Developments
Over the last two years, the HRSA audit has intensified its focus on compliance, duplicate discounts, and diversion prevention. Plus, Executive Order 14297, passed earlier this year, may influence 340B reimbursements and push for greater pricing transparency, mainly affecting hospitals, PBMs, and TPAs that rely on program savings. To effectively optimize program benefits, hospitals, PBMs, and TPAs must move toward digital solutions combined with human oversight to overcome manufacturer restrictions.
Getting Ready for 340B Compliance
340B Drug Pricing Program remains critical for expanding access to care for healthcare providers. However, hospitals, healthcare systems, PBMs, and contract pharmacies are facing increased scrutiny and compliance challenges.
In this situation, effective program management is key to avoiding any potential fines and compliance challenges. Here, our comprehensive end-to-end 340B program management can help you stay compliant and optimize the benefits of your program. So, book your 30-minute free consultation with our 340B experts to discover how we can support your 340B strategy development and implementation.
Frequently Asked Questions
What is the 340B program in health?
The 340 program is a federal US program that allows eligible healthcare providers to purchase outpatient medications at discounted prices, helping offer more affordable care to underserved communities.
What is a 340B pharmacy?
340B pharmacies are those that provide medications purchased through the 340B program, either directly to a healthcare provider or through contract pharmacies.
What is a 340B audit?
HRSA’s 340B program audits review the manufacturer’s compliance with eligibility rules, including 340B program requirements, and ensure that 340B drugs are sold to covered entities at or below the established 340B drug pricing.


 [tta_listen_btn]
[tta_listen_btn]
 November 17 2025
November 17 2025