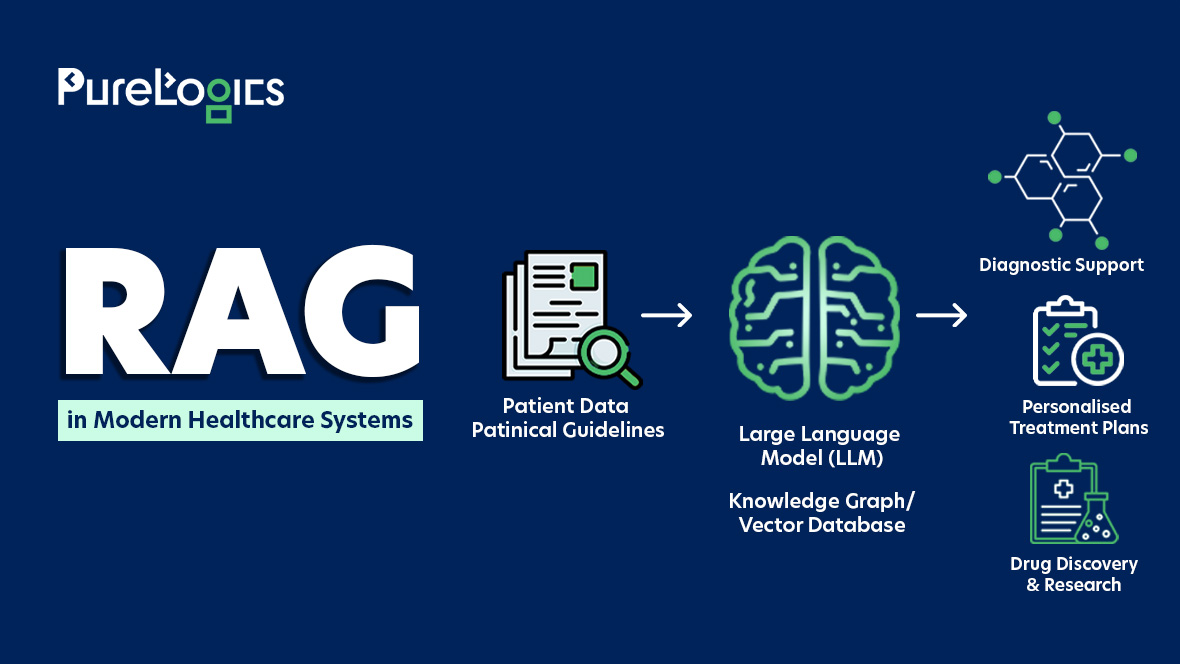
Data privacy can be pretty tricky, especially when you’re trying to make sense of various regulations. Two big names in the world of data privacy are the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA). Although they both aim to safeguard individual privacy, they have different focuses and methods.
For Quality Assurance (QA) professionals, it’s essential to understand these differences to stay compliant. This blog will explore a QA’s perspective on comparing GDPR and HIPAA, pointing out key areas that need careful attention.
Struggling with GDPR or HIPAA compliance?
Stay compliant—explore our healthcare solutions today!
GDPR vs HIPPA- Key Difference
Understanding the intricate world of data privacy regulations is vital for any organization dealing with sensitive information. Two major frameworks in this field are the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA).
Both aim to protect personal data, but they differ in their scopes, requirements, and enforcement methods. Dive into the essential differences between GDPR and HIPAA to see how each regulation influences data handling practices across various sectors:
GDPR
The GDPR, introduced by the European Union (EU) in 2016, is a regulation designed to give individuals control over their personal data while placing stringent requirements on organizations that manage this data. It applies to any company processing data of EU residents, no matter where the organization is based.
HIPPA
HIPAA, a US law established in 1996, aims to protect the privacy of individually identifiable health information, specifically within covered entities. This law applies to healthcare providers, health plans, and healthcare clearinghouses, ensuring that patient information remains confidential and secure.
Side-by-Side: GDPR vs. HIPAA Compliance
Both GDPR and HIPAA focus on protecting personal information, but they differ significantly in their scopes, requirements, and applications. GDPR has a broad reach across multiple sectors within the EU, whereas HIPAA specifically targets the healthcare industry in the United States.
Let’s delve into a comparison of GDPR and HIPAA, emphasizing their key differences and their implications for data privacy and security:
Scope
GDPR protects the personal data of all individuals within the European Union (EU). In contrast, HIPAA safeguards only patients’ healthcare data, known as Protected Health Information (PHI), in the United States.
Consent
GDPR requires explicit user consent for data collection. On the other hand, HIPAA permits some disclosure of PHI for treatment purposes without explicit permission, although informed consent is generally necessary.
Breach Notification
Both regulations mandate data breach notifications. Under HIPAA, notifications must be made within 60 days, whereas GDPR timelines vary depending on the severity of the breach.
Data Subject Rights
GDPR gives individuals “The Right to be Forgotten,” enabling them to request the erasure of their data. In contrast, HIPAA does not provide this right; instead, it requires that medical records be maintained for a specific period.
QA Essentials: Critical Areas to Monitor
Let’s find out the essential QA aspects that demand attention to achieve excellence in software quality:
Scope
GDPR has a wider scope, covering any personal data, whereas HIPAA is specific to protected health information (PHI) within healthcare settings. A QA professional must ensure precise data categorization to identify which regulation applies.
Security Measures
Both regulations require strong security measures to protect data. A QA professional would evaluate the existing security protocols, concentrating on data encryption, access controls, and incident response procedures, to ensure they align with the specific requirements of each regulation.
Breach Notification
Both regulations mandate notifying authorities and individuals in the event of a data breach. A QA professional would review the data breach notification procedures to ensure they adhere to the required timeframes and communication protocols.
Data Subject Rights
The GDPR provides individuals with a broad array of rights concerning their data, such as access, rectification, erasure, and restriction of processing.
HIPAA, on the other hand, grants patients specific rights to access their medical records and request amendments. A QA professional would ensure that processes are in place to efficiently fulfill these rights by the applicable regulation.
Lawful Basis for Executing
Both regulations necessitate a lawful basis for data processing. GDPR provides several bases for processing, while HIPAA depends on specific permitted uses and disclosures of PHI. A QA professional would examine the justification for data processing to ensure it complies with the relevant legal basis.
Developing a QA-Oriented Mindset
An experienced QA professional employs a systematic approach to comparative analysis by following these steps:
Collect Information
Gather all relevant GDPR and HIPAA regulations documentation, including official guidelines and enforcement actions.
Identify Gaps
Compare the requirements of both regulations and identify areas where processes may not align with one or both regulations.
Build Test Cases
Develop test scenarios that mimic real-world data handling practices to evaluate compliance with the requirements of each regulation.
Execute & Analyze
Perform thorough testing and assess the results. Identify any discrepancies and document the necessary corrective actions.
Ongoing Improvements
Quality Assurance is an ongoing process. Continuously review and update procedures to ensure ongoing compliance with evolving regulations.
Safeguarding Data: QA Engineer’s Checklist and Best Practices
Here’s the checklist for QA Engineers to ensure data safety in their projects:
Security Testing
Carry out thorough security and penetration testing to uncover and fix any vulnerabilities.
Password Management
Implement strict password policies that require regular updates, discourage easily guessable passwords, and include multi-factor authentication for added security.
Organizational Procedures
Ensure that access to tools and data is revoked for departing employees. Coordinate efforts between HR and IT to deactivate accounts and secure data during the employee exit process.
Data Breach Responses
Develop a formal incident response plan to effectively detect, respond to, and mitigate data breaches.
Data Gathering & Storage
Ensure user consent is obtained, collect only necessary data, store it securely using encryption, and establish clear timelines for data deletion.
Cookie Management & Session
Make sure that sessions reset after logins and expire after periods of inactivity. Implement secure session management practices to maintain data security.
Documentation & Access Control
Implement access controls with role-based permissions to maintain detailed records of data processing activities, ensuring compliance with GDPR and HIPAA requirements.
Execution Handling & Backup
Set up secure data backups and ensure that no data leaks occur during service disruptions.
Conclusion
Grasping the unique requirements of GDPR and HIPAA is essential for proper data protection and compliance. While both regulations emphasize privacy, they apply to different types of data and sectors.
By concentrating on key areas such as scope, data subject rights, lawful basis for processing, security measures, and breach notifications, QA professionals can ensure strong compliance.
Many countries include health data provisions within their broader data privacy laws, though not all specify detailed measures for protecting this sensitive information. Utilizing a systematic approach to comparative analysis and ongoing improvement will aid organizations in successfully navigating these complex regulatory environments.
PureLogics’ dedicated team possesses substantial expertise in developing comprehensive healthcare solutions. We are more than willing to support you in creating a secure healthcare product. Contact us today.


 [tta_listen_btn]
[tta_listen_btn]
 August 26 2024
August 26 2024